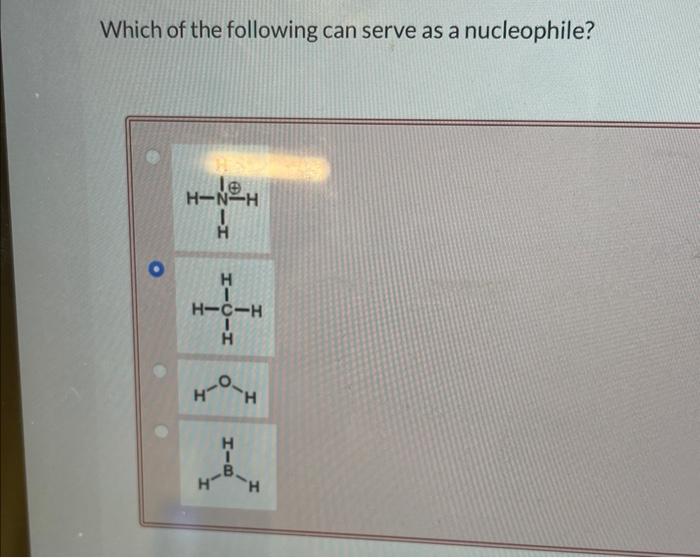
Which of the following can serve as a nucleophile? This captivating inquiry sets the stage for an in-depth exploration into the fascinating realm of nucleophilic chemistry. Nucleophiles, the cornerstone of countless organic reactions, play a pivotal role in shaping the molecular landscape and enabling the synthesis of complex and diverse compounds.
Embarking on this journey, we will unravel the characteristics, reactivity, and applications of nucleophiles, providing a comprehensive understanding of these versatile chemical entities.
Delving into the intricacies of nucleophilicity, we will examine the factors that govern the effectiveness of nucleophiles. Charge, polarity, and steric hindrance emerge as key determinants, influencing the ability of nucleophiles to engage in chemical transformations. Furthermore, we will delve into the mechanisms of nucleophilic reactions, elucidating their role in substitution, addition, and elimination processes.
1. Definition of a Nucleophile: Which Of The Following Can Serve As A Nucleophile
In organic chemistry, a nucleophile is a species that donates an electron pair to an electrophile, forming a new chemical bond. Nucleophiles are typically negatively charged ions or neutral molecules with lone pairs of electrons.
Some common examples of nucleophiles include hydroxide ion (OH-), water (H2O), ammonia (NH3), and alkoxide ions (RO-). These nucleophiles are strong bases and have a high affinity for protons.
2. Common Types of Nucleophiles
There are three main types of nucleophiles:
- Anions:Anions are negatively charged ions that have a high affinity for protons. Some common examples of anions include hydroxide ion (OH-), chloride ion (Cl-), and bromide ion (Br-).
- Lone pairs:Lone pairs are electrons that are not involved in any chemical bonds. They can be found on atoms such as nitrogen, oxygen, and sulfur. Lone pairs are nucleophilic because they can donate their electrons to electrophiles.
- Pi bonds:Pi bonds are double bonds that are formed by the overlap of two p orbitals. Pi bonds can be nucleophilic if they have a high electron density. Some common examples of nucleophilic pi bonds include the pi bonds in alkenes and alkynes.
3. Factors Affecting Nucleophilicity
The nucleophilicity of a species is influenced by several factors, including:
- Charge:Anions are generally more nucleophilic than neutral molecules, and neutral molecules are more nucleophilic than cations.
- Polarity:The polarity of a molecule affects its nucleophilicity. Molecules with a high polarity have a more concentrated electron density on the nucleophilic atom, making them more reactive.
- Steric hindrance:Steric hindrance refers to the presence of bulky groups around the nucleophilic atom. Steric hindrance can reduce the nucleophilicity of a species by making it more difficult for the nucleophile to reach the electrophile.
4. Examples of Nucleophilic Reactions
Nucleophiles are involved in a wide variety of chemical reactions, including:
- Substitution reactions:In a substitution reaction, a nucleophile attacks an electrophile, replacing a leaving group. Substitution reactions are one of the most common types of reactions in organic chemistry.
- Addition reactions:In an addition reaction, a nucleophile adds to an electrophile, forming a new bond. Addition reactions are common in the synthesis of alkenes and alkynes.
- Elimination reactions:In an elimination reaction, a nucleophile removes a proton from an electrophile, causing the elimination of a leaving group. Elimination reactions are common in the synthesis of alkenes and alkynes.
5. Applications of Nucleophiles in Organic Synthesis
Nucleophiles are used in a wide variety of organic synthesis reactions. Some of the most common applications of nucleophiles include:
- Creating new carbon-carbon bonds:Nucleophiles can be used to create new carbon-carbon bonds by reacting with electrophiles. This is a fundamental step in the synthesis of many organic compounds.
- Functional group interconversion:Nucleophiles can be used to interconvert different functional groups. For example, a nucleophile can be used to convert an alcohol into an ether or an ester.
- Protecting groups:Nucleophiles can be used to protect functional groups from unwanted reactions. For example, a nucleophile can be used to protect an amine group from reacting with an electrophile.
Commonly Asked Questions
What is the definition of a nucleophile?
A nucleophile is a chemical species that donates an electron pair to an electrophile, forming a new chemical bond.
What are some common types of nucleophiles?
Common types of nucleophiles include anions, lone pairs, and pi bonds.
What factors affect nucleophilicity?
Factors that affect nucleophilicity include charge, polarity, and steric hindrance.
