In the realm of chemistry, the concept of polarity plays a pivotal role in shaping the behavior of molecules. Polar and nonpolar molecules pogil provides an in-depth exploration of this fascinating topic, delving into the intricacies of molecular interactions and their far-reaching applications.
Polar and nonpolar molecules exhibit distinct properties that influence their solubility, reactivity, and behavior in mixtures. Understanding the polarity of molecules is crucial for comprehending a wide range of chemical phenomena, from the formation of hydrogen bonds to the design of advanced materials.
Polar and Nonpolar Molecules: Polar And Nonpolar Molecules Pogil
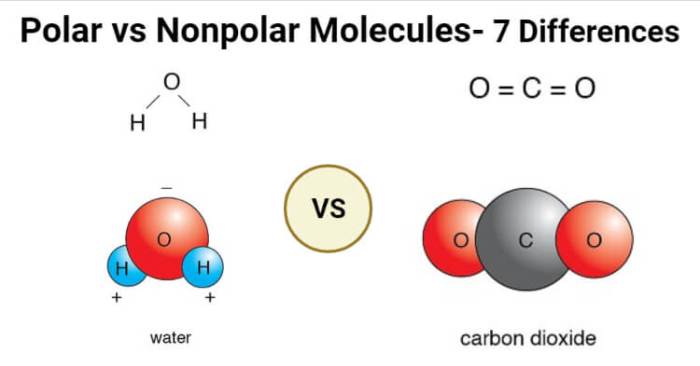
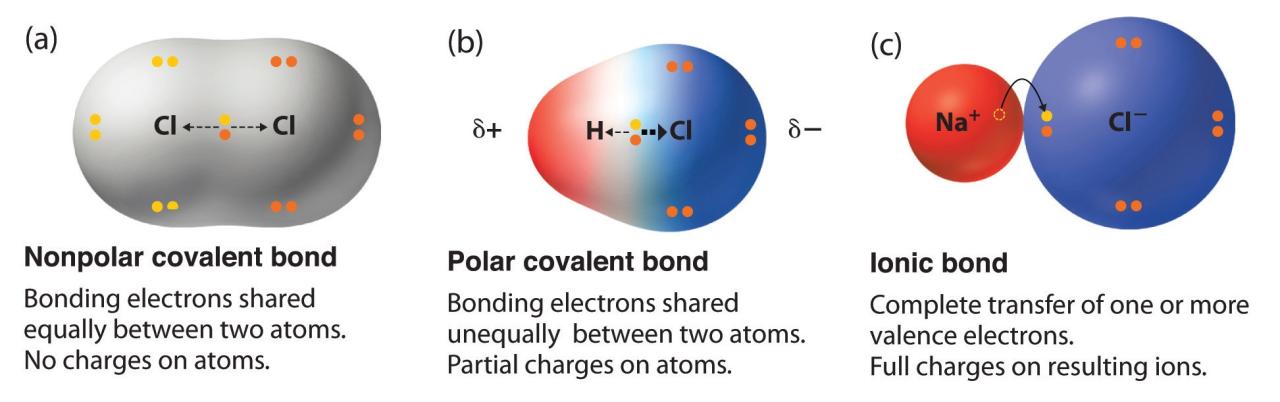
Polar molecules have an uneven distribution of electrons, resulting in a separation of positive and negative charges within the molecule. This polarity arises from differences in electronegativity, the ability of an atom to attract electrons. Nonpolar molecules, on the other hand, have an even distribution of electrons, leading to no net charge separation.
Examples of Polar and Nonpolar Molecules
- Polar molecules:Water (H 2O), ammonia (NH 3), hydrogen chloride (HCl)
- Nonpolar molecules:Methane (CH 4), carbon dioxide (CO 2), hexane (C 6H 14)
Properties and Characteristics
Polar molecules exhibit unique properties compared to nonpolar molecules. Polar molecules tend to be soluble in polar solvents like water, while nonpolar molecules are more soluble in nonpolar solvents like oil. Polarity also influences intermolecular forces, with polar molecules experiencing stronger dipole-dipole interactions and hydrogen bonding compared to nonpolar molecules.
Interactions Between Polar and Nonpolar Molecules
Types of Interactions
- Dipole-dipole interactions:Occur between polar molecules with permanent dipoles.
- Hydrogen bonding:A strong dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms (N, O, F).
- London dispersion forces:Weak interactions that exist between all molecules, regardless of polarity.
Solubility, Polar and nonpolar molecules pogil
Polarity plays a crucial role in determining the solubility of molecules in different solvents. Polar molecules dissolve well in polar solvents due to favorable dipole-dipole interactions and hydrogen bonding. Nonpolar molecules, on the other hand, are more soluble in nonpolar solvents because of the absence of strong intermolecular forces.
Behavior in Mixtures
Polarity affects the behavior of molecules in mixtures. Polar molecules tend to segregate from nonpolar molecules, forming distinct phases. This behavior is observed in mixtures of water and oil, where water molecules cluster together due to their polarity, while oil molecules form a separate layer.
Applications of Polar and Nonpolar Molecules
Chemistry
- Polar solvents are used in various chemical reactions to facilitate the dissolution of polar reactants and promote specific interactions.
- Nonpolar solvents are employed in reactions involving nonpolar compounds and to prevent unwanted interactions with polar reagents.
Biology
- Polarity is essential for the function of biological molecules such as proteins and nucleic acids.
- Nonpolar molecules, like lipids, form the hydrophobic core of cell membranes.
Materials Science
- Polar molecules are used in the design of materials with specific properties, such as hydrogels and ion-exchange resins.
- Nonpolar molecules are utilized in the production of water-resistant materials and lubricants.
Experimental Techniques for Studying Polarity
Dielectric Constant Measurement
This technique measures the ability of a substance to reduce the strength of an electric field. Polar substances have a higher dielectric constant, indicating their ability to reduce the electric field.
Dipole Moment Measurement
This technique involves measuring the torque experienced by a polar molecule in an electric field. The dipole moment is a measure of the polarity of the molecule.
Gas Chromatography
This technique separates molecules based on their polarity. Polar molecules interact more strongly with the stationary phase in the column, leading to longer retention times.
Simulation and Modeling of Polarity
Molecular Dynamics Simulations
These simulations model the interactions between molecules over time. They can provide insights into the behavior of polar and nonpolar molecules in different environments.
Quantum Chemical Calculations
These calculations use quantum mechanics to determine the electronic structure and properties of molecules, including their polarity.
Advantages and Limitations
Simulations and modeling offer valuable insights into the behavior of polar and nonpolar molecules, but they have limitations. They require significant computational resources and may not accurately capture all the complexities of real-world systems.
Frequently Asked Questions
What is the key difference between polar and nonpolar molecules?
Polar molecules have an uneven distribution of charge, while nonpolar molecules have a uniform distribution of charge.
How does polarity affect the solubility of molecules?
Polar molecules are generally more soluble in polar solvents, while nonpolar molecules are more soluble in nonpolar solvents.
What are some applications of polar and nonpolar molecules?
Polar and nonpolar molecules find applications in various fields, including drug design, materials science, and environmental chemistry.