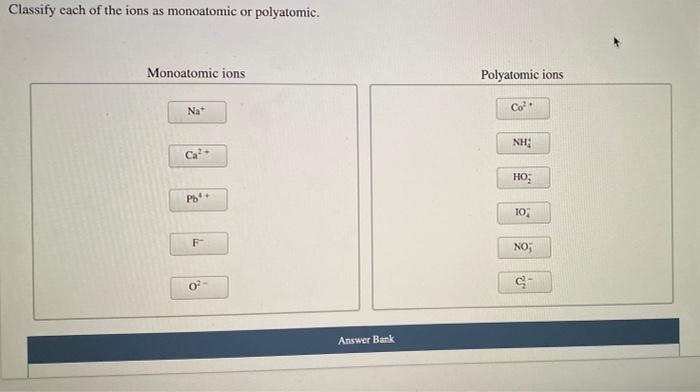
Classify each of the ions as monoatomic or polyatomic, exploring the fundamental differences between these two types of ions. This classification plays a crucial role in understanding chemical reactions and properties, providing a deeper insight into the behavior of matter.
Monoatomic ions, composed of a single atom, stand in contrast to polyatomic ions, which consist of multiple atoms covalently bonded together. Distinguishing between these ions is essential for comprehending their chemical characteristics and reactivity.
Define Monoatomic and Polyatomic Ions
In chemistry, ions are charged particles formed when an atom or molecule gains or loses electrons. Ions can be classified into two types: monoatomic and polyatomic.
Monoatomic ionsare ions that consist of a single atom. They are formed when an atom gains or loses one or more electrons, resulting in a net positive or negative charge. For example, the sodium ion (Na+) is formed when a sodium atom loses one electron, while the chloride ion (Cl-) is formed when a chlorine atom gains one electron.
Polyatomic ionsare ions that consist of two or more atoms that are covalently bonded together. They are formed when a group of atoms gains or loses electrons, resulting in a net positive or negative charge. For example, the sulfate ion (SO42-) is formed when a sulfur atom and four oxygen atoms gain two electrons, while the ammonium ion (NH4+) is formed when a nitrogen atom and four hydrogen atoms gain one electron.
Identify Monoatomic and Polyatomic Ions
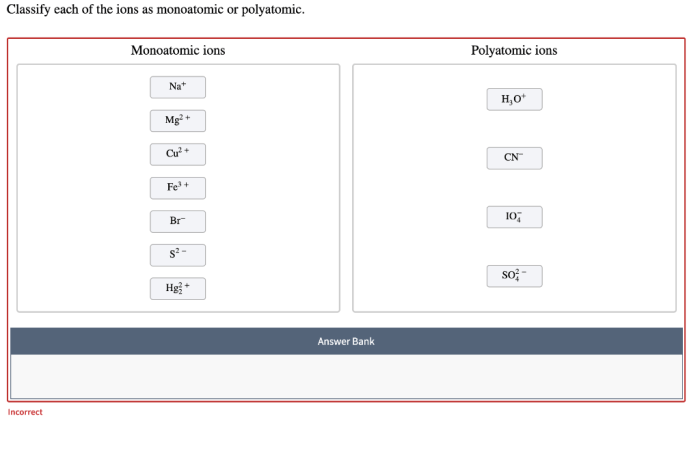
Monoatomic ions can be distinguished from polyatomic ions by their chemical formula. Monoatomic ions have a chemical formula that consists of a single element symbol, while polyatomic ions have a chemical formula that consists of two or more element symbols.
Another way to identify monoatomic and polyatomic ions is by their charge. Monoatomic ions typically have a charge of +1 or -1, while polyatomic ions typically have a charge of +2 or -2.
Classify Ions Using Examples: Classify Each Of The Ions As Monoatomic Or Polyatomic
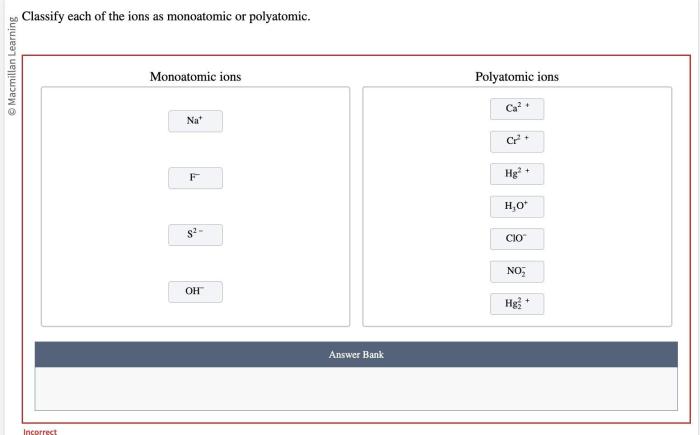
| Ion | Monoatomic/Polyatomic | Explanation |
|---|---|---|
| Na+ | Monoatomic | Sodium atom loses one electron |
| Cl- | Monoatomic | Chlorine atom gains one electron |
| SO42- | Polyatomic | Sulfur atom and four oxygen atoms gain two electrons |
| NH4+ | Polyatomic | Nitrogen atom and four hydrogen atoms gain one electron |
| Ca2+ | Monoatomic | Calcium atom loses two electrons |
| CO32- | Polyatomic | Carbon atom and three oxygen atoms gain two electrons |
| Fe3+ | Monoatomic | Iron atom loses three electrons |
| NO3- | Polyatomic | Nitrogen atom and three oxygen atoms gain one electron |
| K+ | Monoatomic | Potassium atom loses one electron |
| MnO4- | Polyatomic | Manganese atom and four oxygen atoms gain one electron |
Explain the Significance of Ion Classification
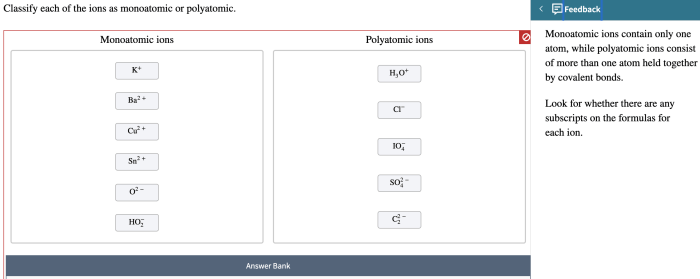
Classifying ions is important in chemistry because it helps us to understand their chemical reactions and properties. For example, monoatomic ions are typically more reactive than polyatomic ions. This is because monoatomic ions have a higher charge density than polyatomic ions, which makes them more likely to react with other ions.
Classifying ions also helps us to understand the properties of ionic compounds. For example, ionic compounds that contain monoatomic ions are typically more soluble in water than ionic compounds that contain polyatomic ions. This is because monoatomic ions are smaller than polyatomic ions, which makes them more likely to dissolve in water.
FAQ Summary
What is the difference between a monoatomic and a polyatomic ion?
Monoatomic ions are composed of a single atom, while polyatomic ions consist of multiple atoms covalently bonded together.
How can I identify monoatomic and polyatomic ions?
Monoatomic ions typically have a single charge, while polyatomic ions have a charge greater than one. Additionally, polyatomic ions often contain oxygen or hydrogen atoms.
Why is it important to classify ions as monoatomic or polyatomic?
Classifying ions helps us understand their structure, bonding, and reactivity. This information is essential for predicting the outcome of chemical reactions and understanding the behavior of matter.

