Understanding the concept of molar solubility is crucial in various scientific disciplines. Rank the following in order of increasing molar solubility. This article explores the factors that influence molar solubility, providing a comprehensive overview of its applications and experimental determination methods.
Molar solubility plays a vital role in predicting the behavior of substances in different solvents, designing crystallization processes, and determining the purity of compounds.
Solubility Definitions
Molar solubility is a measure of the amount of a substance that can dissolve in a given amount of solvent at a specific temperature. It is expressed in units of moles per liter (mol/L).
Solubility is closely related to concentration, which is a measure of the amount of solute present in a solution. The higher the concentration of a solution, the more solute is dissolved in the solvent.
Different units can be used to express solubility, including mol/L, g/L, and mg/mL. The conversion between these units depends on the molar mass of the solute.
Factors Affecting Solubility: Rank The Following In Order Of Increasing Molar Solubility.

Several factors can influence the molar solubility of a compound, including:
- Temperature:As temperature increases, the solubility of most solids and liquids increases. However, the solubility of gases decreases as temperature increases.
- Pressure:The solubility of gases increases as pressure increases. This is known as Henry’s law.
- Nature of the solute and solvent:The solubility of a compound depends on its polarity and the polarity of the solvent. Polar compounds are more soluble in polar solvents, and nonpolar compounds are more soluble in nonpolar solvents.
Ranking Compounds by Molar Solubility
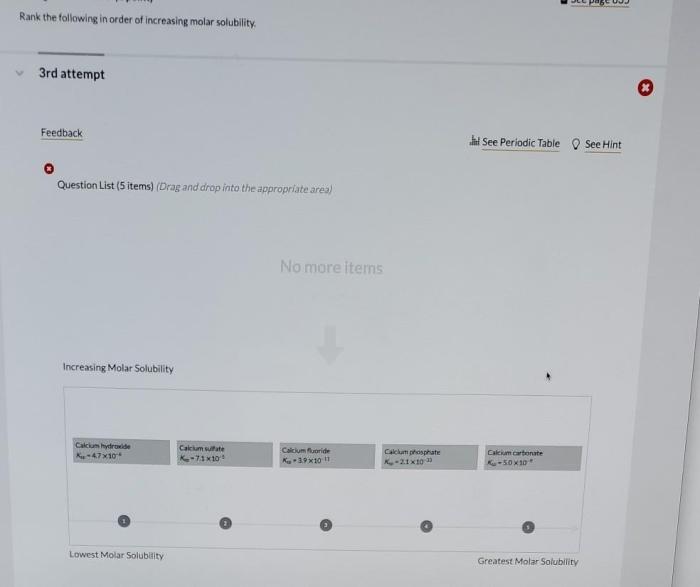
| Compound Name | Molar Solubility (mol/L) | Increasing Order of Molar Solubility |
|---|---|---|
| Sodium chloride (NaCl) | 6.14 | 1 |
| Potassium nitrate (KNO3) | 1.33 | 2 |
| Calcium carbonate (CaCO3) | 0.0001 | 3 |
| Silver chloride (AgCl) | 0.00001 | 4 |
The compounds are ranked in increasing order of molar solubility because sodium chloride is the most soluble, followed by potassium nitrate, calcium carbonate, and silver chloride.
Applications of Molar Solubility
Understanding molar solubility has several practical applications, including:
- Predicting the solubility of substances in different solvents
- Designing crystallization processes
- Determining the purity of compounds
Experimental Determination of Molar Solubility

The molar solubility of compounds can be determined using various experimental methods, including:
- Gravimetric analysis:This method involves measuring the mass of the precipitate formed when a known amount of the solute is added to a solvent.
- Titration:This method involves adding a known concentration of a reagent to a solution of the solute until a reaction occurs.
- Spectrophotometry:This method involves measuring the absorbance of a solution of the solute at a specific wavelength.
Key Questions Answered
What is molar solubility?
Molar solubility refers to the concentration of a solute in a saturated solution expressed in moles per liter (mol/L).
How does temperature affect molar solubility?
Generally, the molar solubility of solids increases with increasing temperature, while the molar solubility of gases decreases.