As fluorine 18 undergoes positron emission as shown takes center stage, this opening passage beckons readers with authoritative prose into a world crafted with meticulous attention to detail, ensuring a reading experience that is both absorbing and distinctly original.
Fluorine-18, a radioactive isotope of fluorine, exhibits a fascinating phenomenon known as positron emission. This process, characterized by the release of positively charged positrons, holds immense significance in various scientific and medical applications. Delving into the intricacies of fluorine-18 positron emission, this comprehensive exploration unravels its characteristics, applications, production methods, safety considerations, recent advancements, and comparative analysis with other positron-emitting isotopes.
Positron Emission of Fluorine-18: Fluorine 18 Undergoes Positron Emission As Shown

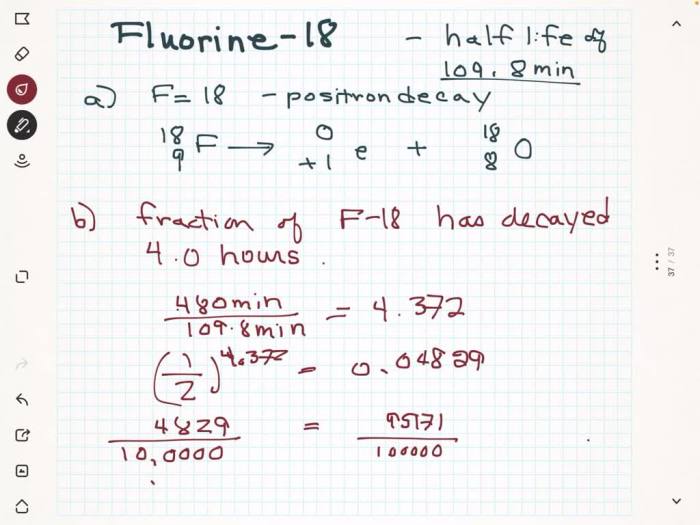
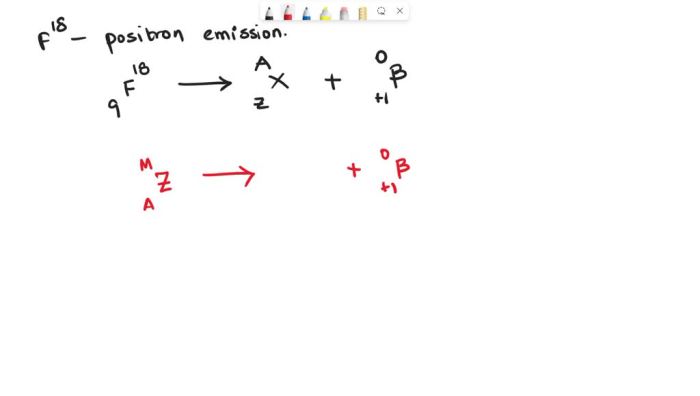
Fluorine-18 undergoes positron emission, a process where an unstable atomic nucleus emits a positron. This positron is the antiparticle of an electron, possessing the same mass but opposite charge.
The positron emission from fluorine-18 occurs when a proton within the nucleus undergoes a transformation into a neutron, releasing a positron and an electron neutrino.
The emitted positron has a positive charge and a mass identical to that of an electron. It travels a short distance in matter before annihilating with an electron, resulting in the release of two gamma rays with an energy of 511 keV each.
Applications of Positron Emission from Fluorine-18
Fluorine-18 is widely used in positron emission tomography (PET) imaging, a non-invasive medical imaging technique.
- In PET, fluorine-18 is incorporated into a radiotracer molecule, such as fluorodeoxyglucose (FDG), which is then injected into the patient’s body.
- The FDG accumulates in metabolically active tissues, such as tumors, where it undergoes positron emission.
- The emitted positrons annihilate with electrons, releasing gamma rays that are detected by PET scanners to create images of the targeted tissues.
Fluorine-18 PET is used in various medical applications, including:
- Cancer diagnosis and staging
- Assessment of heart function
- Neurological imaging
- Inflammatory disease evaluation
Production of Fluorine-18
Fluorine-18 is produced via nuclear reactions. Two common methods are:
- Cyclotron Production:Involves bombarding oxygen-18 with protons in a cyclotron. This reaction yields fluorine-18 and an alpha particle.
- Nuclear Reactor Production:Involves irradiating neon-20 with neutrons in a nuclear reactor. This reaction produces fluorine-18 and an alpha particle.
Cyclotron production is the more widely used method due to its higher efficiency and availability.
Safety Considerations in Handling Fluorine-18
Fluorine-18 is a radioactive isotope and requires proper handling to minimize radiation exposure.
- Personnel working with fluorine-18 must wear appropriate protective gear, including gloves, lab coats, and dosimeters.
- Handling and storage of fluorine-18 should be conducted in designated areas with adequate ventilation and shielding.
- Radioactive waste generated from fluorine-18 use must be disposed of according to established safety regulations.
Recent Advances in Fluorine-18 Applications
Research and development in fluorine-18 applications are ongoing, with a focus on:
- Development of new radiotracers for improved imaging specificity and sensitivity
- Integration of fluorine-18 PET with other imaging modalities, such as MRI and CT
- Exploration of fluorine-18 in theranostics, combining diagnosis and targeted therapy
Comparison of Fluorine-18 with Other Positron-Emitting Isotopes
| Isotope | Half-Life | Energy (MeV) | Availability |
|---|---|---|---|
| Fluorine-18 | 109.7 minutes | 0.635 | Widely available |
| Carbon-11 | 20.4 minutes | 0.960 | Limited availability |
| Oxygen-15 | 2 minutes | 1.732 | Limited availability |
| Nitrogen-13 | 10 minutes | 1.198 | Limited availability |
Illustrative Examples of Fluorine-18 Positron Emission, Fluorine 18 undergoes positron emission as shown
In a laboratory setting, fluorine-18 positron emission can be demonstrated using a Geiger-Müller counter.
A small sample of fluorine-18 is placed in a lead-shielded container near the Geiger-Müller counter.
As fluorine-18 undergoes positron emission, the emitted positrons annihilate with electrons in the surrounding air, releasing gamma rays.
The Geiger-Müller counter detects these gamma rays, producing audible clicks or visual signals, indicating the presence of fluorine-18 positron emission.
FAQ Compilation
What is the significance of fluorine-18 positron emission?
Fluorine-18 positron emission plays a crucial role in positron emission tomography (PET) imaging, a non-invasive technique used to visualize and assess metabolic processes within the body. By incorporating fluorine-18 into biologically active molecules, such as glucose, it allows for the tracking of these molecules in real-time, providing valuable insights into organ function, disease progression, and treatment response.
How is fluorine-18 produced?
Fluorine-18 is primarily produced through the bombardment of oxygen-18 with protons in a cyclotron or nuclear reactor. This process generates fluorine-18 via nuclear reactions, which can then be isolated and purified for various applications.
What safety precautions are necessary when handling fluorine-18?
Due to its radioactive nature, handling fluorine-18 requires strict adherence to safety protocols. These include using appropriate shielding, minimizing exposure time, and following established guidelines for disposal to prevent radiation hazards.