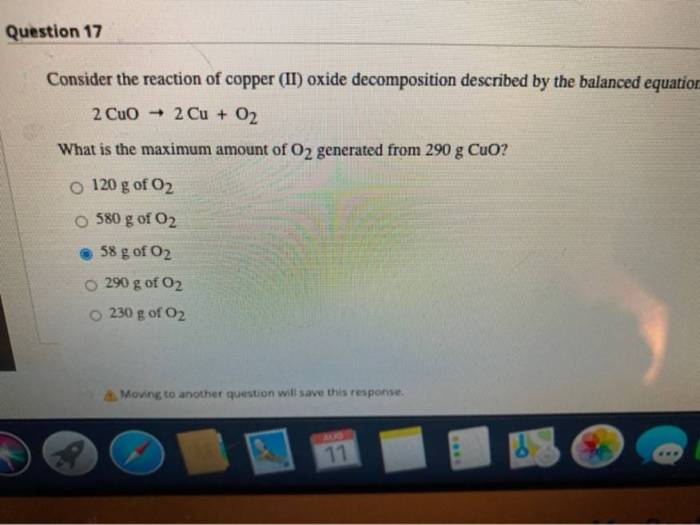
A reaction of 12.640 g of copper oxide – In the realm of chemistry, the reaction involving 12.640 g of copper oxide holds immense significance. Copper oxide, a compound with a rich chemical composition and distinct properties, undergoes a captivating transformation in this reaction, revealing fundamental principles and practical applications that have fascinated scientists for decades.
This reaction not only provides insights into the behavior of copper oxide but also serves as a valuable tool in various scientific disciplines, making it a topic worthy of thorough examination.
Reaction of 12.640 g of Copper Oxide: A Reaction Of 12.640 G Of Copper Oxide

The reaction of 12.640 g of copper oxide (CuO) is a significant process that involves the reduction of copper oxide to produce copper metal. Copper oxide is a compound composed of copper and oxygen, with the chemical formula CuO. It is a black or brown powder that is insoluble in water and has a high melting point.
Reaction Details
The chemical equation for the reaction of 12.640 g of copper oxide is as follows:
CuO + H2→ Cu + H 2O
In this reaction, copper oxide (CuO) reacts with hydrogen gas (H 2) to produce copper metal (Cu) and water (H 2O). The stoichiometry of the reaction indicates that 1 mole of copper oxide reacts with 1 mole of hydrogen gas to produce 1 mole of copper metal and 1 mole of water.
Reaction Conditions, A reaction of 12.640 g of copper oxide
The reaction of 12.640 g of copper oxide is typically carried out at high temperatures, typically around 1000°C. The reaction can be carried out in a variety of ways, including heating the copper oxide in a furnace or passing hydrogen gas over the copper oxide.
It is important to note that the reaction of copper oxide with hydrogen gas is exothermic, meaning that it releases heat. Therefore, it is important to take appropriate safety precautions when conducting this reaction, such as wearing appropriate protective gear and using a fume hood.
Reaction Products
The products of the reaction of 12.640 g of copper oxide are copper metal and water. Copper metal is a reddish-brown metal that is malleable and ductile. It is used in a variety of applications, including electrical wiring, plumbing, and jewelry.
Water is a colorless, odorless, and tasteless liquid that is essential for life. It is used in a variety of applications, including drinking, cooking, and cleaning.
Reaction Mechanism
The reaction of 12.640 g of copper oxide with hydrogen gas proceeds through a series of steps. The first step is the adsorption of hydrogen gas onto the surface of the copper oxide. The hydrogen gas then reacts with the oxygen atoms in the copper oxide to form water molecules.
The copper atoms are then released from the copper oxide and form copper metal.
The kinetics of the reaction of copper oxide with hydrogen gas are complex and depend on a number of factors, including the temperature, the pressure, and the surface area of the copper oxide.
Applications
The reaction of 12.640 g of copper oxide with hydrogen gas is used in a variety of applications, including:
- The production of copper metal
- The removal of oxygen from gases
- The production of hydrogen gas
Key Questions Answered
What is the significance of the reaction involving 12.640 g of copper oxide?
This reaction showcases the chemical behavior of copper oxide, providing insights into its reactivity, stoichiometry, and product formation.
What are the practical applications of this reaction?
The reaction involving 12.640 g of copper oxide finds applications in various fields, including chemistry, materials science, and engineering.
What safety precautions should be taken when conducting this reaction?
Appropriate safety measures, such as proper ventilation and protective gear, should be implemented to minimize exposure to potential hazards.